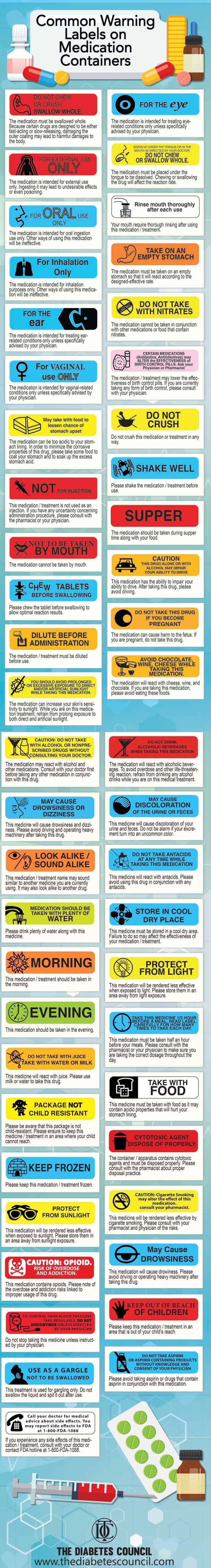
38 cautionary and advisory labels for dispensed medicines
Guidance for cautionary and advisory labels | About | BNF | NICE Wordings which can be given as separate warnings are labels 1-19, 29-30, and 32. Wordings which can be incorporated in an appropriate position in the directions for dosage or administration are labels 21-28. A label has been omitted for number 20; labels 31 and 33 no longer apply to any medicines in the BNF and have therefore been deleted. Poisons Standard June 2021 - Legislation 5/26/2021 · “Required Advisory Statements for Medicine Labels” means the document made under subsection 3 ... where the cautionary statement “POSSESSION WITHOUT AUTHORITY ILLEGAL” is required by Section 1.3(1)(b), ... 1.5.6 Dispensed medicines (1) Unless otherwise specified by regulation: ...
FDA adds Boxed Warning for risk of serious injuries caused by ... 2/18/2022 · [04-30-2019] The Food and Drug Administration (FDA) is advising that rare but serious injuries have happened with certain common prescription insomnia medicines because of sleep behaviors ...

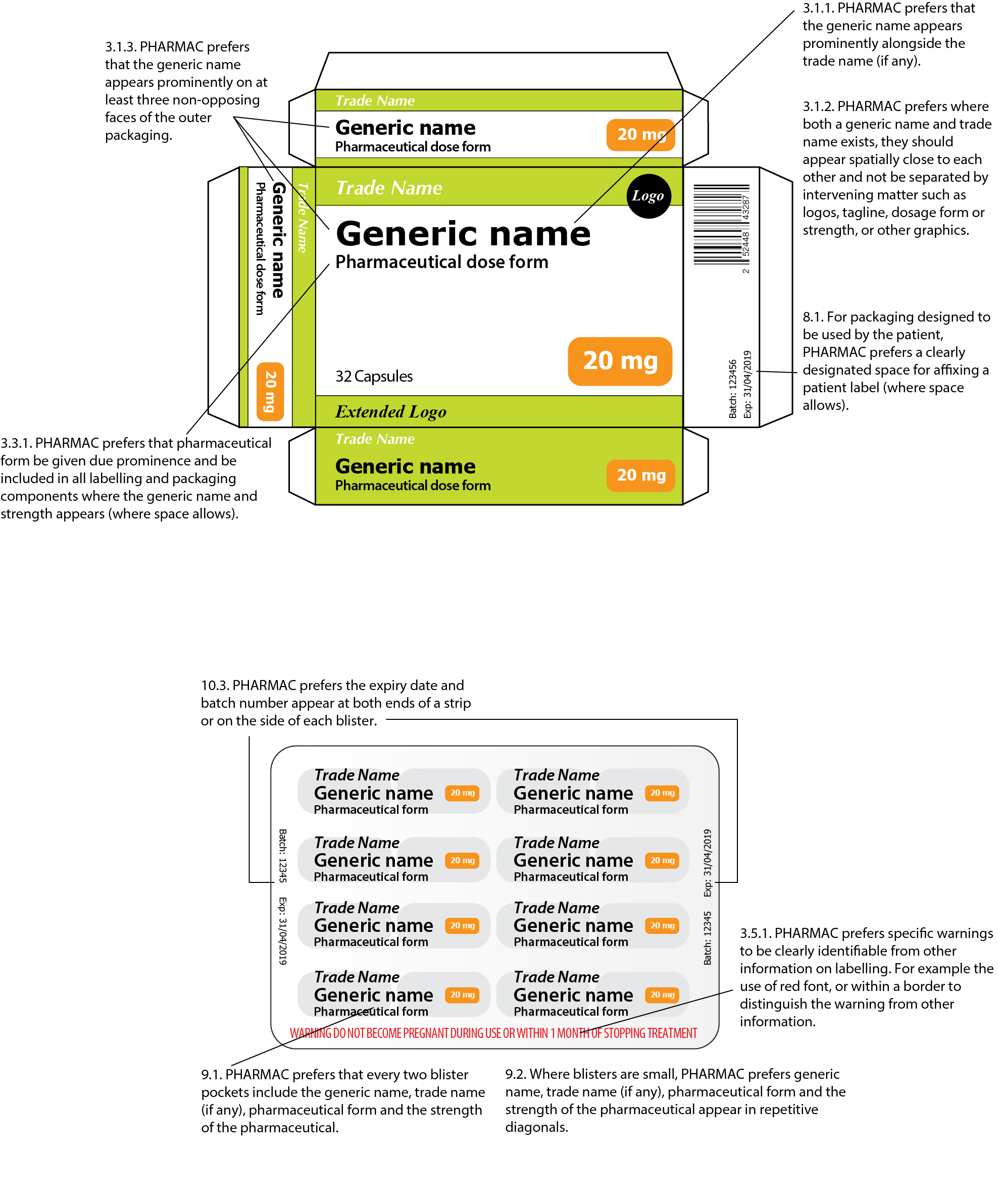
Cautionary and advisory labels for dispensed medicines
Auxiliary label - Wikipedia An auxiliary label (also called cautionary and advisory label or prescription drug warning label) is a label added on to a dispensed medication package by a pharmacist in addition to the usual prescription label. These labels are intended to provide supplementary information regarding safe administration, use, and storage of the medication. Auxiliary labels provide information which can ... Labels on medicines and poisons - Department of Health Some medicines also require additional label warnings. For example, oral retinoids must have warnings about becoming pregnant. Sedation warnings. Medicines listed in Appendix K of the SUSMP (exernal site) must be labelled with a sedation warning when supplied to patients. Pharmacy cautionary advisory label 1 or label 1A should be used. About - BNF Publications As such, BNF Publications content also indirectly reflect patient views from the perspective of the source material. The only BNF Publications content directly aimed at patients is the Cautionary and Advisory warnings that appear on labels of medicines dispensed to patients. All of these labels were user-tested by patients during development.
Cautionary and advisory labels for dispensed medicines. Cautionary and advisory labels for medicines - Everything2.com These are the code numbers and their meanings for the cautionary labels used by pharmacist s when dispensing medicine s in the UK. Extra counselling may be given with relation to age, experience , background and understanding of the patient. Warning. May cause drowsiness British National Formulary - Wikipedia The British National Formulary (BNF) is a United Kingdom (UK) pharmaceutical reference book that contains a wide spectrum of information and advice on prescribing and pharmacology, along with specific facts and details about many medicines available on the UK National Health Service (NHS). Information within the BNF includes indication(s), contraindications, side effects, doses, … › Details › F2022L00730Poisons Standard June 2022 - Legislation May 31, 2022 · Human medicines required to be labelled with a sedation warning. List of human medicines required to be labelled with a warning regarding their sedation potential. Appendix L. Requirements for dispensing labels for medicines. Requirements applying to labels attached to medicines at the time of dispensing. Appendix M Public Health Preparedness Capabilities: National Standards for State ... In 2011, CDC established 15 capabilities that serve as national standards for public health preparedness planning. Since then, these capability standards have served as a vital framework for state, local, tribal, and territorial preparedness programs as they plan, operationalize, and evaluate their ability to prepare for, respond to, and recover from public health emergencies.
› aboutAbout - BNF Publications As such, BNF Publications content also indirectly reflect patient views from the perspective of the source material. The only BNF Publications content directly aimed at patients is the Cautionary and Advisory warnings that appear on labels of medicines dispensed to patients. All of these labels were user-tested by patients during development. Poisons Standard June 2022 - Legislation 5/31/2022 · “Required Advisory Statements for Medicine Labels” means the document made under subsection 3 ... where the cautionary statement “POSSESSION WITHOUT AUTHORITY ILLEGAL” is required by Section 1.3(1)(b), ... 1.5.6 Dispensed medicines (1) Unless otherwise specified by regulation: ... Warning statements for labels and leaflets of certain medicines Details. Warning statements need to be added to the labels and leaflets of certain medicines. The words in this guidance do not need to be used verbatim but that have already been user tested and ... › sonushanno › community-pharmacyCommunity Pharmacy - SlideShare Aug 09, 2016 · 4. Alternative medicines- In some countries, pharmacists supply traditional medicines and dispense homoeopathic prescriptions Checking symptoms of minor aliments- pharmacist can supply a non-prescription medicine, with advice to consult a medical practitioner if the symptoms persist for more than a few days. Alternatively, the pharmacist may ...
bnf.nice.org.uk › about › labelsCautionary and advisory labels | About | BNF | NICE To be used on preparations containing ofloxacin and some other quinolones, doxycycline, lymecycline, minocycline, and penicillamine. These drugs chelate calcium, iron, and zinc and are less well absorbed when taken with calcium-containing antacids or preparations containing iron or zinc. Cautionary and advisory labels for dispensed medicines - Blogger Cautionary and advisory labels for dispensed medicines Numbers following the preparation entries in the BNF correspond to the code numbers of the cautionary labels that pharmacist are recommended to add when dispensing. It is also expected that pharmacists will counsel patients when necessary. Cautionary and Advisory Labels for Dispensed Medicines Cautionary and Advisory Labels for Dispensed Medicines. Pharmaceutical Society of New Zealand, 1988 - Drugs - 30 pages. 0 Reviews. Reviews aren't verified, but Google checks for and removes fake content when it's identified. What people are saying - Write a review. We haven't found any reviews in the usual places. PDF cautionary advisory labels - Openbook Howden use of medicines, and provide optimal outcomes for consumers. Reproduced with the permission of the Pharmaceutical Society of Australia. Sold in dispenser boxes of 1000. The cautionary advisory labels (CALs) have become an essential part of Australian pharmacy practice. cautionary advisory labels 2-14 Paul Street St Marys SA 5042
Cautionary and advisory labels | About | BNF | NICE Medicines Information Services; Labels; Guidance for cautionary and advisory labels; On this page. Label 1; Label 2; Label 3; Label 4; ... To be used on containers of dispensed solid dose preparations containing paracetamol for adults when the instruction on the label indicates that the dose can be taken on an ‘as required’ basis. The dose ...
The Drugs and Cosmetics Rules, 1945 - Indian Kanoon 4/7/2016 · An application for an import licence shall be made to the licensing authority in Form 8 for drugs excluding those specified in Schedule X, and in Form 8A for drugs specified in Schedule X, either by the manufacturer himself having a valid wholesale licence for sale or distribution of drugs under these rules, or by the manufacturer’s agent in India either having a valid licence …
Bagot Press - Pharmacy Consumables & Print Specialists Cautionary and Advisory Labels The Bagot Press Cautionary and Advisory label system is one of the few approved licences used for printing labels for prescribed medicines and the bright colour labels draw attention to important specific information to patients. Ordering Guide
Cautionary and advisory labels for medicines - SlideShare 1 of 18 Cautionary and advisory labels for medicines Nov. 12, 2013 • 11 likes • 10,711 views Download Now Download to read offline Health & Medicine Business presentation on commonly used advisory labels on medication and need of this Kiran Sharma Follow Assistant Professor at KIET Ghaziabad Advertisement More Related Content
Safer dispensing labels for prescription medicines the specific legislative requirements for dispensing labels provided by pharmacists, prescribers, nurse practitioners and dentists are defined in state-based regulations and are informed by the poisons standard. 17 the pharmacy board of australia's guidelines for dispensing of medicines provides best-practice guidance for the labelling of …
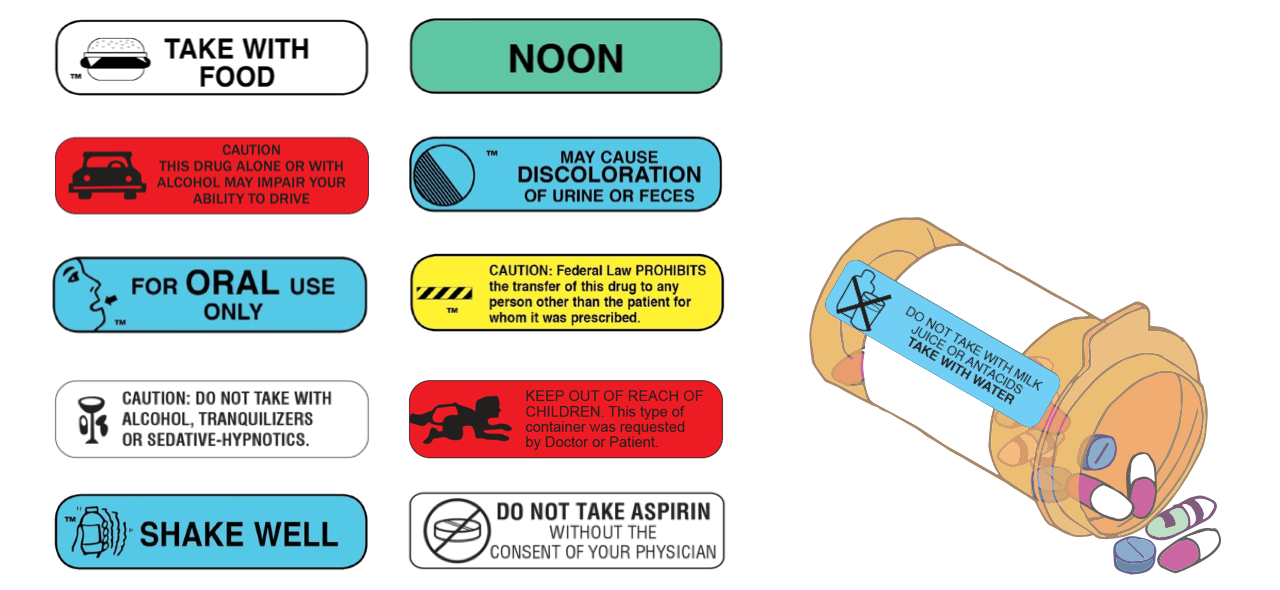
Cautionary and Advisory Label | Cautionary Advisory Label A dispensing label is always added to a medicine to show the essential details (the name of the medicine, the dose, and the frequency of administration), and in some cases the pharmacist will add cautionary and advisory labels. Standard cautionary and advisory labels offer advice but are not exhaustive.
› Details › F2021L00650Poisons Standard June 2021 - Legislation May 26, 2021 · Human medicines required to be labelled with a sedation warning. List of human medicines required to be labelled with a warning regarding their sedation potential. Appendix L. Requirements for dispensing labels for medicines. Requirements applying to labels attached to medicines at the time of dispensing. Appendix M
Cautionary and Advisory Label | Cautionary Advisory Label | Technology ... A dispensing label is always added to a medicine to show the essential details (the name of the medicine, the dose, and the frequency of administration), and in some cases the pharmacist will add cautionary and advisory labels. Standard cautionary and advisory labels offer advice but are not exhaustive.
Community Pharmacy - SlideShare 8/9/2016 · Cautionary and advisory labels for medicines Kiran Sharma. Featured (20) Irresistible content for immovable prospects ... supervision of a pharmacist where the practice of pharmacy occurs or where prescription orders are compounded and dispensed other than a hospital pharmacy or a limited service pharmacy. Hospital pharmacy Hospital pharmacy is ...
indiankanoon.org › doc › 16293633The Drugs and Cosmetics Rules, 1945 - Indian Kanoon Apr 07, 2016 · An application for an import licence shall be made to the licensing authority in Form 8 for drugs excluding those specified in Schedule X, and in Form 8A for drugs specified in Schedule X, either by the manufacturer himself having a valid wholesale licence for sale or distribution of drugs under these rules, or by the manufacturer’s agent in India either having a valid licence under the ...
Cautionary_and_advisory_label - chemeurope.com Cautionary and advisory label Cautionary and advisory labels (Cals) are sometimes added (with the dispensing label) to a medicine dispensed by the pharmacist to the patient. Weighing the right way Daily Sensitivity Test Recognize and detect the effects of electrostatic charges on your balance
Join LiveJournal Password requirements: 6 to 30 characters long; ASCII characters only (characters found on a standard US keyboard); must contain at least 4 different symbols;
Cautionary_and_advisory_label : definition of Cautionary_and_advisory ... Cautionary and advisory labels (Cals) are sometimes added (with the dispensing label) to a medicine dispensed by the pharmacist to the patient. A dispensing label is always added to a medicine to show the essential details (the name of the medicine, the dose, and the frequency of administration), and in some cases the pharmacist will add cautionary and advisory labels.
› createJoin LiveJournal Password requirements: 6 to 30 characters long; ASCII characters only (characters found on a standard US keyboard); must contain at least 4 different symbols;
Cautionary advisory labels - Australian Pharmacist The dispensing pharmacist has dispensed the patient's discharge medications and has labelled simvastatin with cautionary advisory label 21 (Special handling and disposal required - ask your pharmacist) and label A (Swallow whole do not crush or chew). You are concerned that these labels may alarm and/or confuse the patient. What should you do?
About - BNF Publications As such, BNF Publications content also indirectly reflect patient views from the perspective of the source material. The only BNF Publications content directly aimed at patients is the Cautionary and Advisory warnings that appear on labels of medicines dispensed to patients. All of these labels were user-tested by patients during development.
Labels on medicines and poisons - Department of Health Some medicines also require additional label warnings. For example, oral retinoids must have warnings about becoming pregnant. Sedation warnings. Medicines listed in Appendix K of the SUSMP (exernal site) must be labelled with a sedation warning when supplied to patients. Pharmacy cautionary advisory label 1 or label 1A should be used.
Auxiliary label - Wikipedia An auxiliary label (also called cautionary and advisory label or prescription drug warning label) is a label added on to a dispensed medication package by a pharmacist in addition to the usual prescription label. These labels are intended to provide supplementary information regarding safe administration, use, and storage of the medication. Auxiliary labels provide information which can ...






























Post a Comment for "38 cautionary and advisory labels for dispensed medicines"